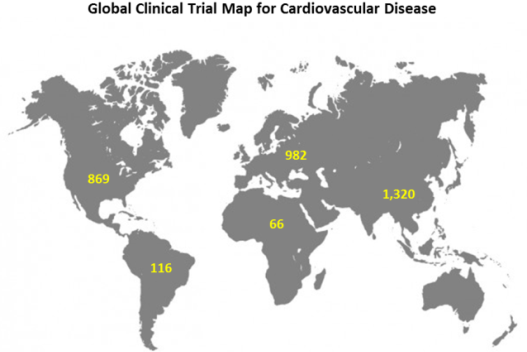
AdisInsight shows 10,115 clinical studies investigating pharmaceutical treatment of cardiovascular disease being conducted in 144 countries. Some of these studies will be active in either the planning, recruitment, or administration stage while other will be inactive either completed or discontinued. The clinical trial map shown below aggregates information across all trials researching cardiovascular disease to show where active clinical studies are taking place at a regional level.
Read more
One of those clinical studies taking place in Austria, Belgium, Canada, Denmark, England, France, Germany, Italy, Netherlands, Norway, Poland, Portugal, Scotland, Spain, Sweden, United Kingdom, and USA is a phase III, multicenter, rRandomized, double-blind placebo-controlled study to assess the efficacy and safety of tocilizumab in subjects with giant cell arteritis. This trial sponsored by Roche is active and no longer recruiting subjects. Results from this study were presented at the American College of Rheumatology (ACR) and Association for Rheumatology Health Professionals (ARHP) Annual Meeting and published in the New England Journal of Medicine. The trial met the primary endpoint with a significant increase in the proportion of patients achieving sustained remission at one year (56% in the weekly treatment group [QW; p < 0.0001] and 53.1% in the bi-weekly treatment group [Q2W; p < 0.0001]) versus 14% in a six-month steroid taper regimen given alone. The secondary endpoint were also met with a significant increase in the proportion of patients achieving sustained remission at one year (56% [QW; p < 0.0001] and 53.1% [Q2W; p = 0.0002]) compared to 17.6% with a 12-month steroid taper regimen given alone. The study also demonstrated that tocilizumab had a generally well tolerated adverse events (AEs) profile and the safety profile of the tocilizumab groups was generally consistent with the documented safety profile of tocilizumab in other indications. Incidence of AEs was similar in the 4 treatment arms. No deaths or new vision loss incidents were reported over the period of observation. Fewer patients reported serious adverse events in the tocilizumab weekly (15%) and biweekly groups (14.3%) than in the placebo combined with a 26-week steroid taper regimen (22%) and placebo combined with a 52-week steroid taper regimen groups (25.5%). Below is an excerpt from the AdisInsight trial profile for this study.


